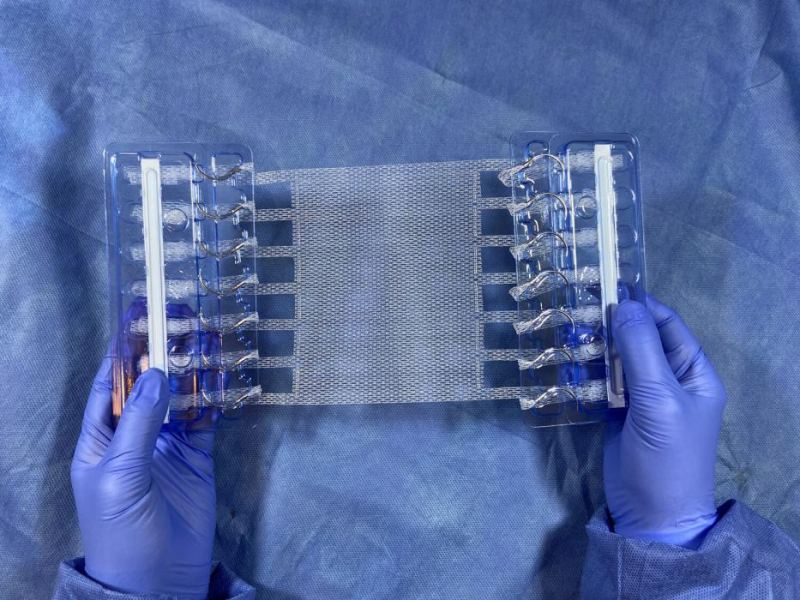
A Duke startup, Deep Blue Medical Advances, has received FDA clearance to start selling its patented T-Line® Hernia Mesh product, which "solves the issue of failure of mesh at fixation sites in ventral hernia repair," according to the company's website.
The company was founded in 2014 by Dr. Howard Levinson, Associate Professor of Surgery in the Duke School of Medicine.
In 2016, Levinson received a Transformative Research award from Duke CTSI Accelerator to complete bench-top and in vivo studies of the hernia mesh—an early investment that is now paying off as the product can begin to improve the lives of patients.
From WRAL TechWire:
"The T-Line Hernia Mesh “provides superior anchoring strength and eliminates a key point of failure for conventional mesh fixation,” the Durham-based company says. It is designed to counter “mesh migration, contraction and eventual failure.”
Millions of hernia surgeries are done globally at billions in clinical costs and support a $1.1 billion hernia device market."
In addition to funding and project management from CTSI Accelerator, Deep Blue benefitted from Duke's translational research pipeline, receiving commercialization support through the Duke Office of Licensing and Ventures.